
客服热线
400-801-9858(工作日08:30-17:00)
邮箱
info@anlongen.com
Since the outbreak of the novel coronavirus nCoV, Anlong Gene raced against time, quickly completed the research and development of Detection Kit.Our partners returned to work during the Spring Festival, making full efforts to production and helping epidemic prevention and control work.
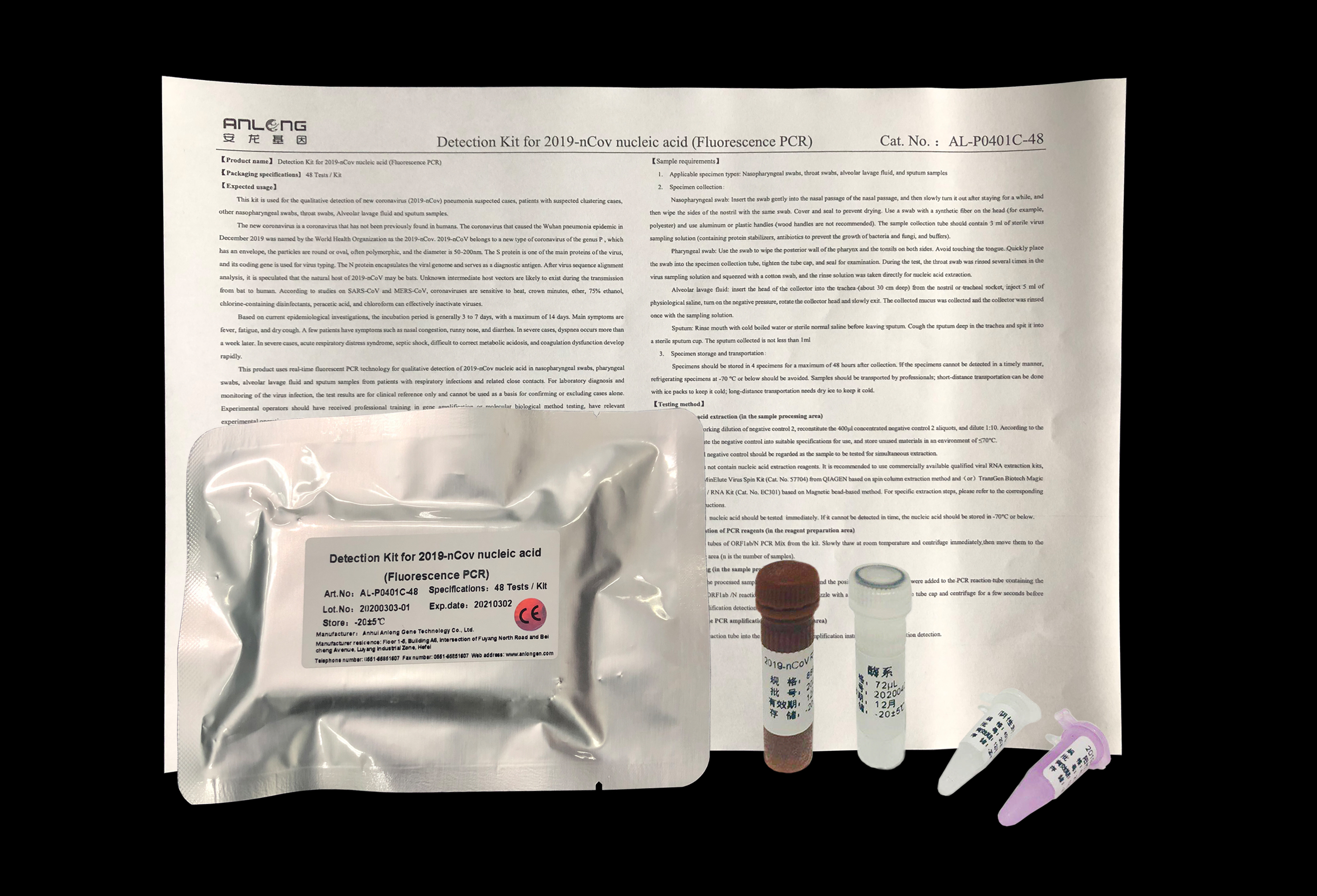
In March 12, 2020, The Detection Kit for 2019-nCov nucleic acid (Fluorescence PCR) of Anhui Anlongen Gene Technology Co., Ltd. had passed the European CE certification,and the daily output can reach 200000 tests.

On June 3th, the detection kit was officially entered ‘the List of medical material manufacturers certified or registered by foreign standards’published on the official website of China Chamber of Commerce for import and export of medical and health products.
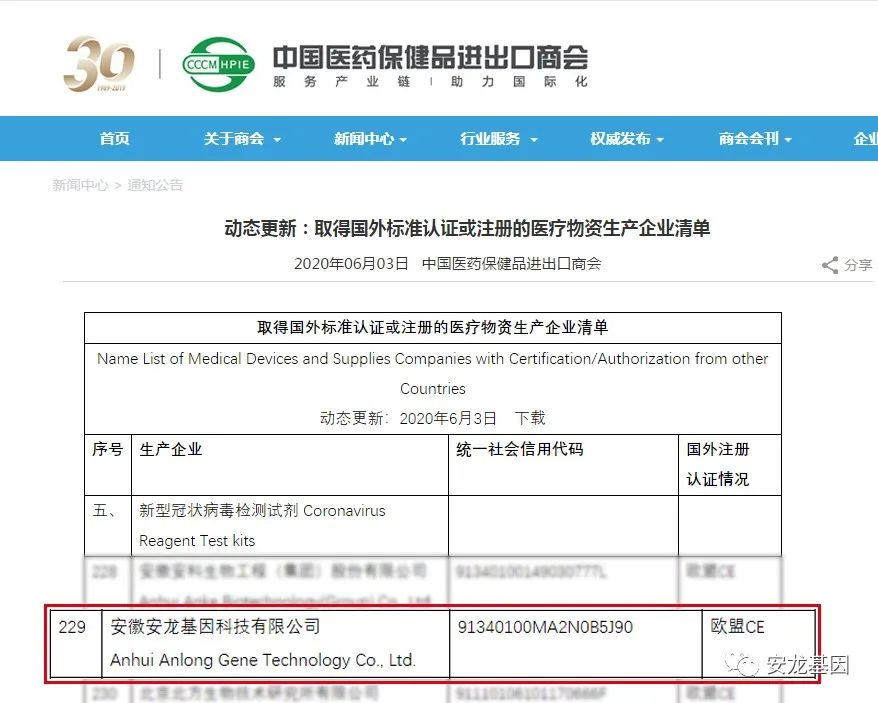
Anlong gene will continue to support the global anti epidemic with a high sense of social responsibility!

敬请期待……